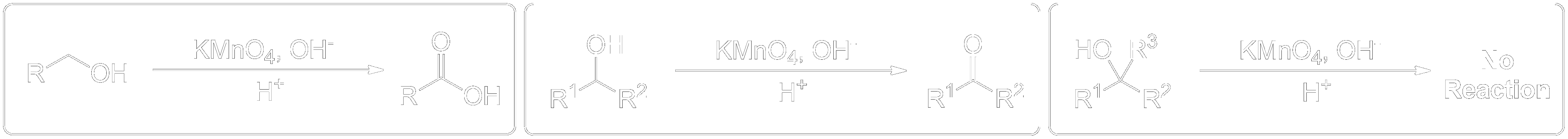
 Potassium Permanganate can be used to oxidize primary alcohols to carboxylic acids and secondary alcohols to ketones. This method is harsh and is also suitable for acidic harsh conditions and acid sensitive compound conditions. 1
Potassium Permanganate can be used to oxidize primary alcohols to carboxylic acids and secondary alcohols to ketones. This method is harsh and is also suitable for acidic harsh conditions and acid sensitive compound conditions. 1
Summary
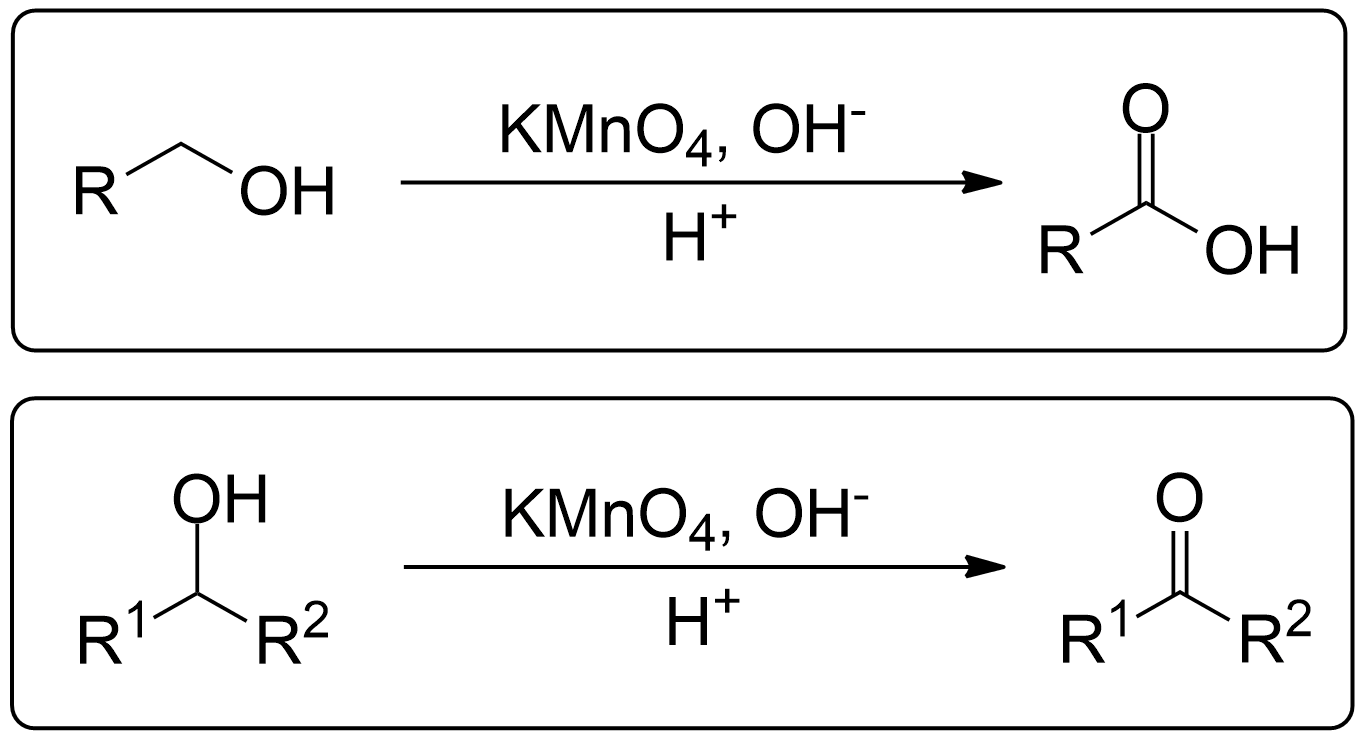
The reaction entry summary. Find the general scheme and full summarized mechanisms here.General Scheme
This section briefly summarizes what can and cannot undergo reactions.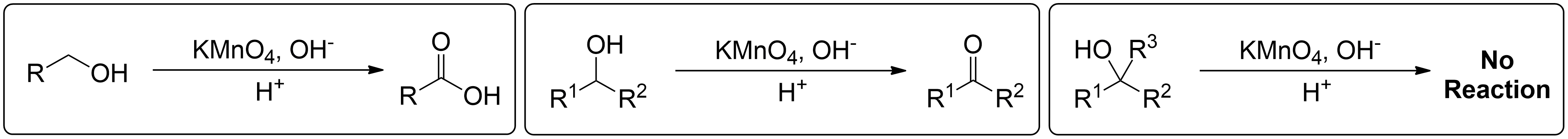
- 1° Alcohols (Primary) get oxidized to Carboxylic Acids.
- 2° Alcohols (Secondary) get oxidized to Ketones.
- 3° Alcohols (Tertiary) do not get oxidized at all.
Other Conditions
- Potassium Permanganate can be used alongside sulfuric acid to oxidize 1° Alcohols (Primary) to Carboxylic Acids
General Mechanism
This section briefly summarizes steps to find the product and perform the mechanisms. Quick steps to finding the product for any alcohol- Identify the reagents.
- Assign side chains (non alcohol part).
- Selectively convert Alcohol to correct product based on alcohol type. Nothing else.
- Keep the side chains (non alcohol part) the same and piece together the full molecule together again.
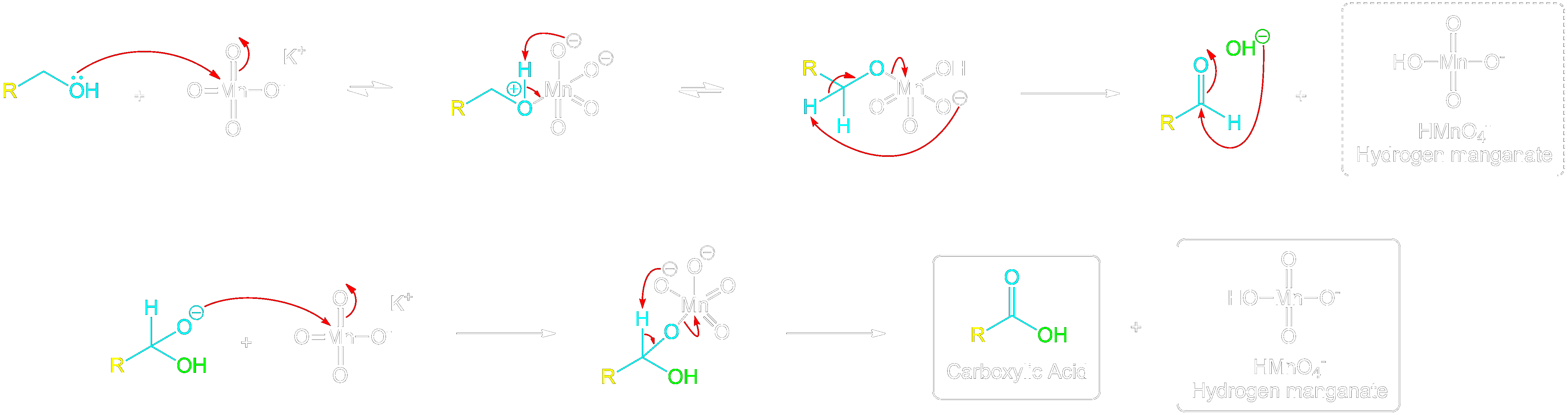
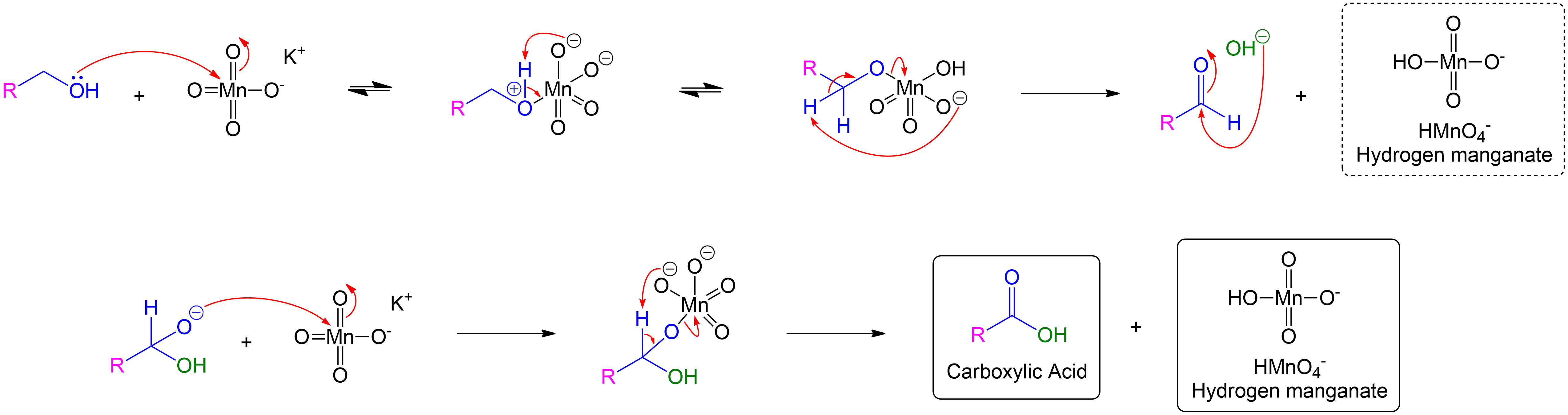
Full Primary Alcohol to Carboxylic Acid Mechanism
Full Primary Alcohol to Carboxylic Acid Mechanism
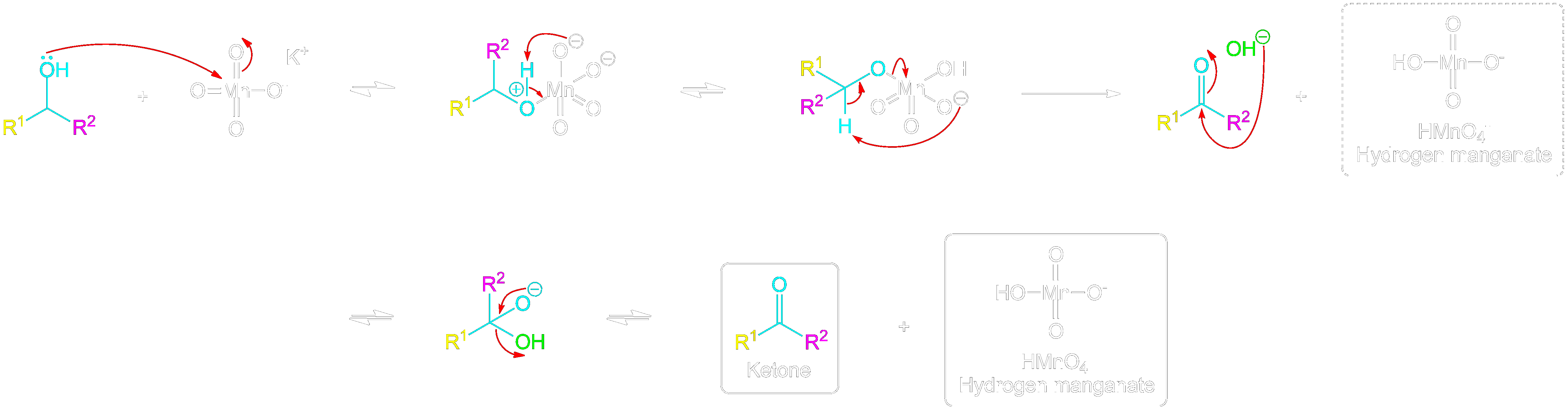
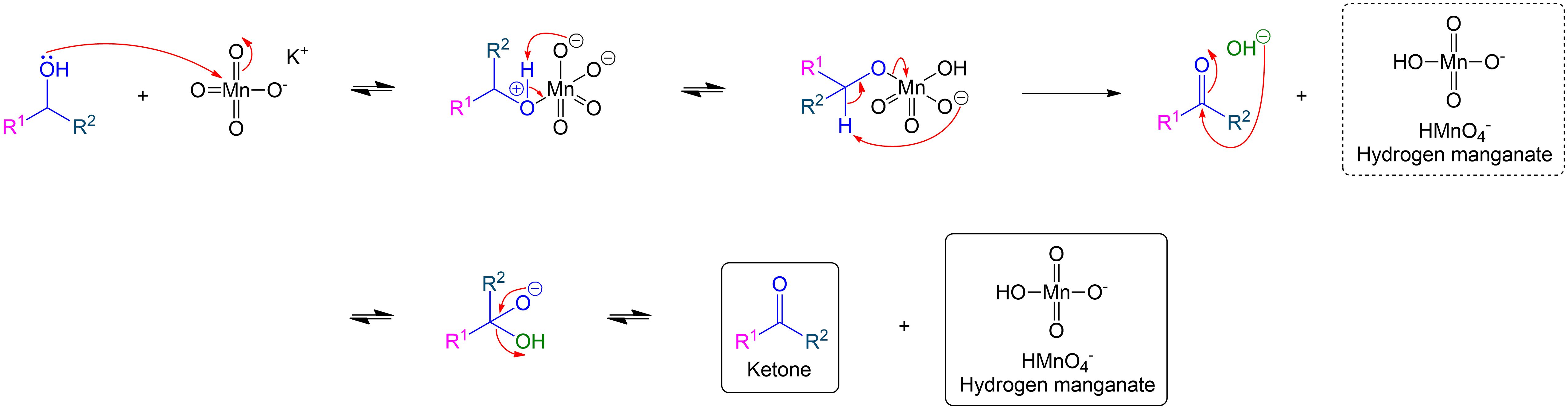
Full Secondary Alcohol to Ketone Mechanism
Full Secondary Alcohol to Ketone Mechanism
References
- Shaabania, A.; Mirzaeia, P.; Naderia, S.; Lee, D. G. Permanganate supported on active manganese dioxide can be used effectively for the oxidation of arenes, alcohols and sulfides under heterogeneous or solvent-free conditions. Tetrahedron 2004, 60, 11415–11420. DOI: 10.1016/j.tet.2004.08.081